Pro-ocular™
Join Our Mission
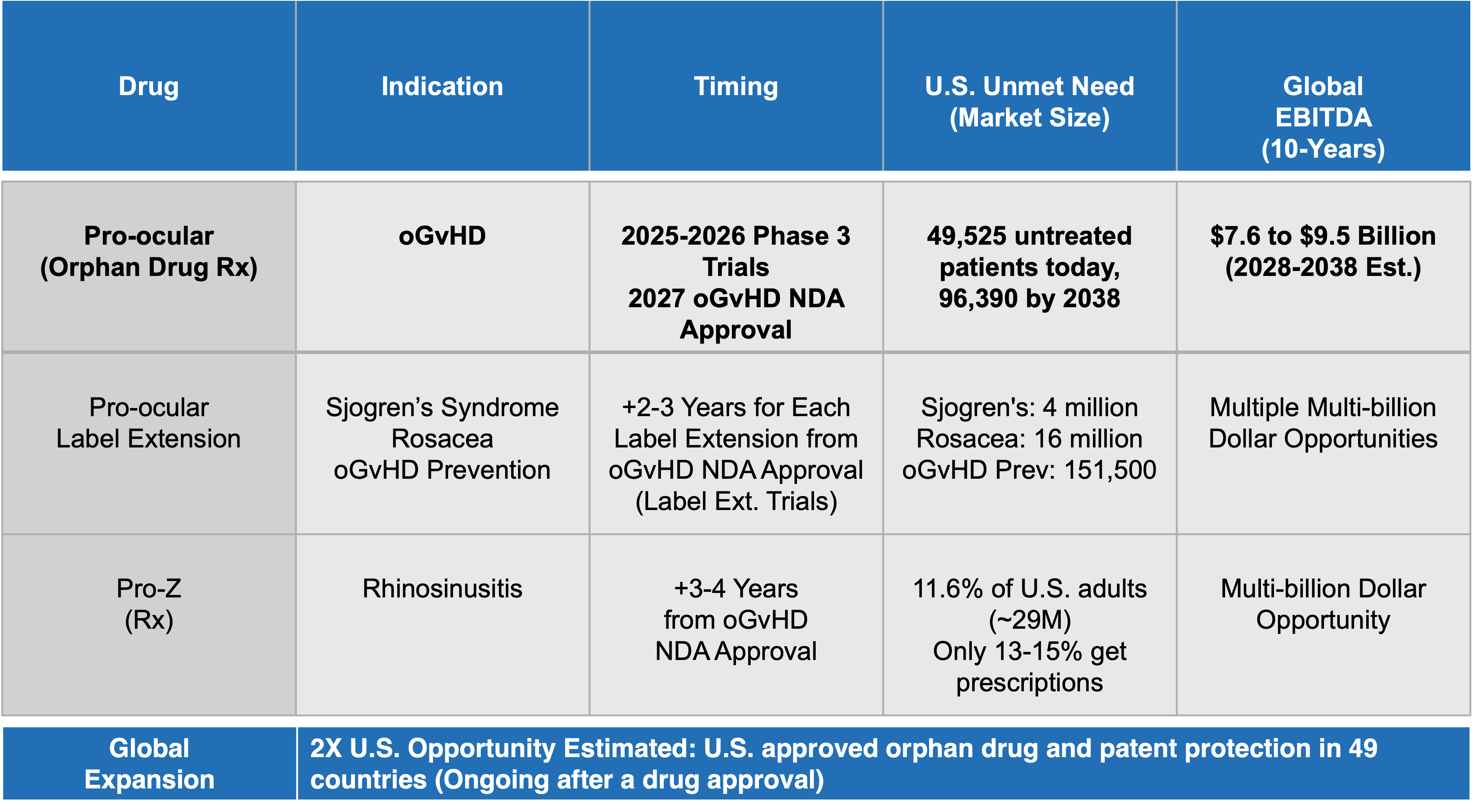
Signal12 is planning to facilitate Phase 3 clinical trials in 2025 and bring Pro-ocular™ to market.
![]()
Signal12 Inc.
Signal12 is a Phase 3-ready ophthalmic drug company. Our proprietary topical drug, Pro-ocular™, has proven safe and effective in Phase 2 clinical studies, demonstrating significant reductions in the signs and symptoms of ocular Graft-versus-Host Disease (oGvHD)—a chronic, debilitating condition following an allogeneic stem cell transplant. Pro-ocular™ is the only effective treatment for oGvHD, employing transappendageal delivery to precisely target the ophthalmic branch of the trigeminal nerve
Signal12 is actively raising capital to complete its Phase 3 clinical trials and bring Pro-ocular™ to market. For information on investment opportunities, please email Neil Edwards at [email protected]. The company has offices in Greenwich, CT and Boston, MA.
© 2025 Signal 12 Inc. | Email: [email protected]